On May 20, 2021 Health Canada opened a consultation on its proposal to modernize the regulatory framework for clinical trials related to human drugs, medical devices, non-prescription drugs, and natural health products to seek feedback from key stakeholders to validate and inform further policy development. This future policy is part of the Targeted Regulatory Review – Regulatory roadmap development under the Regulatory Review of Drugs and Devices (R2D2) initiative.
What changes are proposed to the clinical trials regulation?
To achieve a very ambitious goal of clinical trials regulatory modernization, there is a need for significant change to the whole ecosystem of clinical research. This significant change is exactly what Health Canada is trying to achieve by proposing a single clinical trial framework for all health products. This framework will lay out a foundation for a new regulatory regime that would provide proportional risk-based oversight with a single authorization of a trial, regulatory flexibility over the lifecycle of the trial, greater transparency, enhanced technology to improve patient recruitment, and a modernized compliance and enforcement regime. The schematics of the new approach is presented in Figure 1 below. (Source: Health Canada)
Figure 1
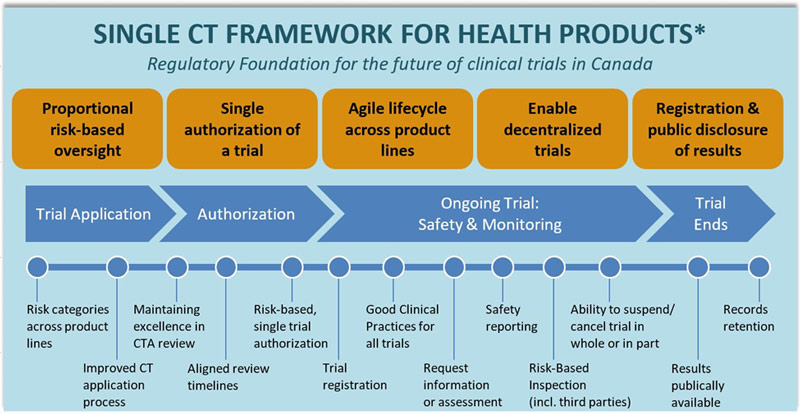
Health Canada proposes modernization to 4 major areas, presented in Figure 2 and Table 1. The biggest impact will be implementation of the risk-based approach, where trials that fall under Category A could be exempt from the authorization process. This will be consistent with the current process, for example, exemption of Phase IV clinical trial from Health Canada review. Category B will allow authorization with tailored requirements for the drugs and devices where safety information is available. For example, a marketed product that is tested for a non-authorized indication. The high risk devices, new drugs, and new NHPs will fall under Category C and will require authorization with full requirements.
As a part of implementation of risk-based approach, Health Canada would also have authority to impose terms and conditions to mitigate significant risks related either to the product or to the conduct of the trial. These terms and conditions could include, but are not limited to, more frequent safety reporting and special population monitoring. The terms and conditions approach will be applied on a case-by-case basis and will be especially critical for an advanced therapeutic product with a novel mode of action and unknown safety profile.
The modernization of clinical trial regulations would not be complete without consideration for patients. Canada is a vast country with dispersed population. This has a significant impact on the recruitment for clinical trials and also the ability of patients across Canada to have access to innovative therapies through clinical trials. As such, Health Canada intends to enable and encourage the conduct of decentralized clinical trials (DCT), where patients could participate in the trial remotely without frequent travel to the clinical site. The regulatory proposal to enable this approach includes but is not limited to:
- Change the wording in the regulations from "written informed consent" to "documented informed consent". This change could lead to implementation of an electronic consent process.
- Allow for a witness to attest that informed consent was given.
- Define "trial site" in the regulation as "The location(s) where trial-related activities are actually conducted", where "trial related activities" could include recruitment, informed consent, monitoring, and visits that are done in a virtual manner.
- Allow greater flexibility for the types of health professionals that can perform the role of a Qualified Investigator
In the next few years, the clinical trials regulatory framework in Canada will change fundamentally and we have an opportunity to be active participants in defining and implementing this change.
About the Author
Oxana Iliach, PhD
Sr. Director, Regulatory Strategy and Policy at Certara/Synchrogenix
Dr. Iliach has more than 15 years of experience in the healthcare industry including the last 10+ years in regulatory affairs. She has expertise in developing and executing regulatory strategies for drugs for rare diseases, pediatrics, and biosimilars, with a focus on Chemistry, Manufacturing and Control (CMC). Oxana has experience with the FDA, EMA, Health Canada, and other smaller agencies. She has a MSc in Chemistry and PhD in Pharmaceutical Science. She is also a professor at Seneca College, Toronto, Canada, where she teaches a course on clinical trials regulations.
References:
- Health Canada. Clinical Trials Modernization: Consultation Paper. https://www.canada.ca/en/health-canada/programs/consultation-clinical-trials-regulatory-modernization-initiative/document.html
- Health Canada. Consultation: Health Canada's Clinical Trials Regulatory Modernization Initiative. https://www.canada.ca/en/health-canada/programs/consultation-clinical-trials-regulatory-modernization-initiative.html
- Health Canada. Health and Biosciences: Targeted Regulatory Review – Regulatory Roadmap. https://www.canada.ca/en/health-canada/corporate/about-health-canada/legislation-guidelines/acts-regulations/targeted-regulatory-reviews/health-biosciences-sector-regulatory-review/roadmap.html#a4
- O Iliach. Health Canada Regulatory Modernization: Yesterday, Today and Tomorrow. White Paper. https://www.certara.com/app/uploads/2021/03/WP_Health_Canada_Regulatory_Modernization.pdf
